Latest News and Tweets
October 2022

Sound Life Sciences acquired by Google
The former ENACT partner was purchased in October... ...(more)
May 2022

BARDA Strategic Plan 2022 - 2026
BARDA Strategic Plan 2022 - 2026 ...(more)
March 2022

Accelerating innovations in kidney disease to improve health equity and outcomes
Accelerating innovations in kidney disease ...(more)

FDA Clears the Biobeat Remote Patient Monitoring Device and Platform for Additional Vital Signs
FDA Clears the Biobeat Remote Patient Monitoring Device ...(more)
February 2022

Aidar Health one of ten startups selected for the M2D2 IMPACT Accelerator Cycle 2 Cohort
Aidar Health one of ten startups selected ...(more)
December 2021
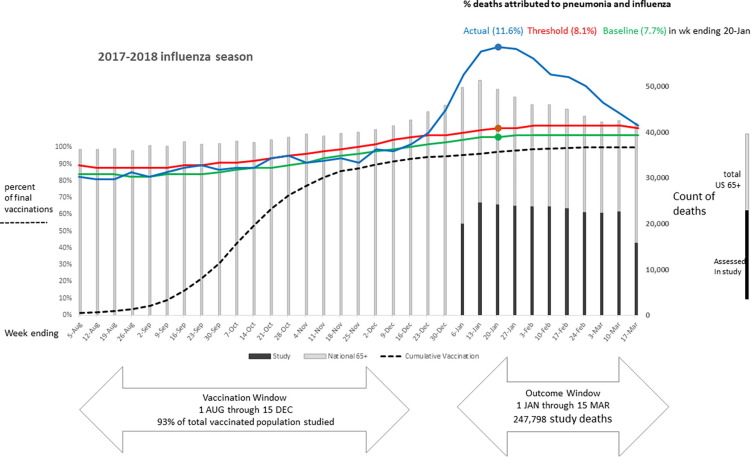
Seasonal influenza vaccination is associated with reduced risk of death among Medicare beneficiaries
Seasonal influenza vaccination is associated with ...(more)

Sound Life Science gets FDA clearance for the first sonar-based respiratory monitor for smart devices
Sound Life Science gets FDA clearance ...(more)
November 2021

Using epigenetics to objectively map diseases and develop new therapeutics
A recent study (1) published in the Journal of the American Medical Association (JAMA), entitled, “Racism, Not Race, Drives Inequality Across the COVID-19 Continuum,”...(more)
October 2021

RizLab Health chosen as one of ten companies for IndieBio NY’s Accelerator Class 3
RizLab Health chosen as one of ten companies ...(more)

BARDA seeks applications for developing at-home COVID-19 diagnostics and technologies
Now accepting submissions to develop home-based, over-the-counter diagnostics for the detection of SARS CoV-2 and technologies to support and improve at-home testing....(more)

The Health Blog is Live!
BLUE KNIGHT™: Building the Shield to Protect Vulnerable Populations. ...(more)
September 2021

BARDA and Partner Therapeutics collaborate on development of a novel assay to stratify sepsis patients that may benefit from an immune therapeutic
BARDA is collaborating with Partner Therapeutics (PTx) to advance a new diagnostic approach to stratify sepsis patients who are most likely to benefit from...(more)
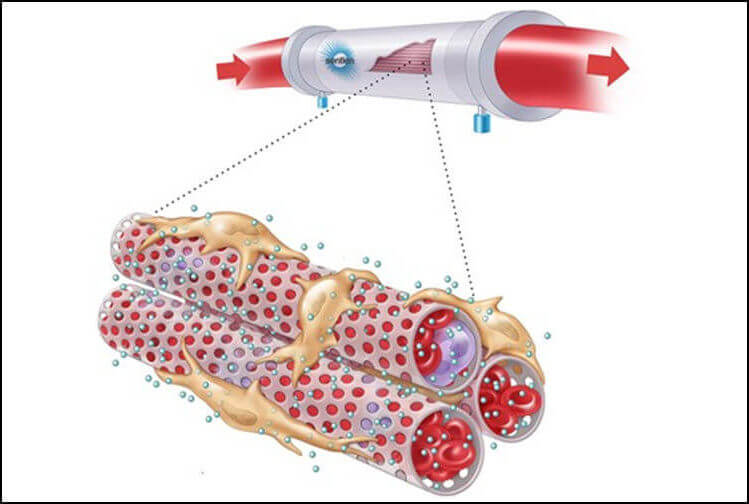
BARDA and Sentien Biotechnologies partner on a novel ex vivo cell-based therapy to reduce disease severity in sepsis patients
BARDA is collaborating with Sentien Biotechnologies to evaluate the company’s cell-based therapeutic platform in people with sepsis, initially focusing on those with acute kidney injuries associated with sepsis (sepsis-AKI), including from COVID-19. ...(more)

BARDA DRIVe evolves its ENACT program into two areas of disruptive technology development of purely digital health tools and at-home diagnostic testing
BARDA's Division of Research, Innovation, and Ventures (DRIVe) today expanded the Early Notification to Act, Control, and Treat (ENACT) program to drive disruptive technologies ...(more)
August 2021

BARDA seeks partners to develop agnostic diagnostic assays
On August 23, 2021, BARDA's Division of Research, Innovation, and Ventures (DRIVe) opened a new area of interest (AOI) under the EZ Broad Agency Announcement (EZ-BAA) solicitation to expand the capabilities ...(more)

US Biologic® and BARDA DRIVe Partner to Develop Oral Flu Vaccines for Home Delivery
US Biologic today announces a partnership with BARDA DRIVe to develop oral influenza vaccines designed to increase access to these life-saving technologies...(more)
June 2021

MediciNova Initiates Sheep Study under Partnership with BARDA to Develop MN-166 (ibudilast) as a Medical Countermeasure Against Chlorine Gas-induced Lung Injury
MediciNova has partnered with the Biomedical Advanced Research and Development Authority (BARDA), part of the ...(more)
May 2021
Maternal Sepsis Week - Raise Awareness, Save Moms
Maternal Sepsis Week is an annual observance that raises awareness of the unique signs and symptoms of sepsis during the pregnancy and postpartum ...(more)

Cytovale Study Establishes Cytovale IntelliSep Test in the Rapid Diagnosis of Sepsis
Development and validation of a cellular host response test as an early diagnostic for sepsis ...(more)
March 2021

Aura receives first of its kind European CE mark for early detection of COVID-19
After years of hard work, we are excited to announce that Empatica has received the CE mark for its respiratory infection detection ...(more)

BARDA and MediciNova, Inc. partner to repurpose MN-166 (ibudilast) as a medical countermeasure against chlorine exposure
During a chemical emergency, the ability to use commonly available medical countermeasures (MCM) can help save lives...(more)
January 2021

A Day On, Not a Day Off – MLK Day of Service
The 2021 Martin Luther King Jr. Day of Service is almost here – and people across the country are rolling up their sleeves in service to fight COVID-19...(more)
December 2020
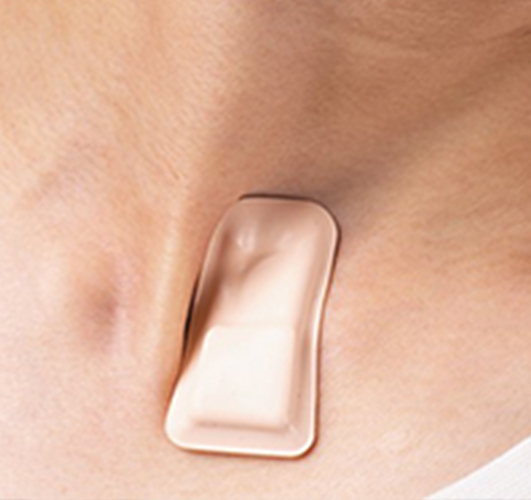
Wearable COVID-19 Sensor Receives Major Award from US Department of Defense
Northwestern University researchers have put their wearable COVID-19 monitoring device through a flurry of tests, updates ...(more)
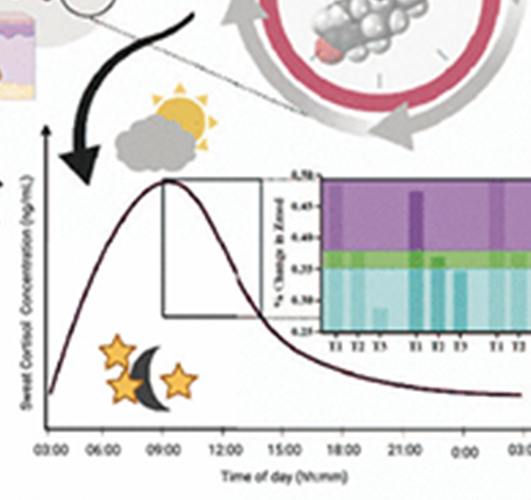
Autonomous, Real-Time Monitoring Electrochemical Aptasensor for Circadian Tracking
The proposed work involves the development of an autonomous, label-free electrochemical sensor for real-time ...(more)
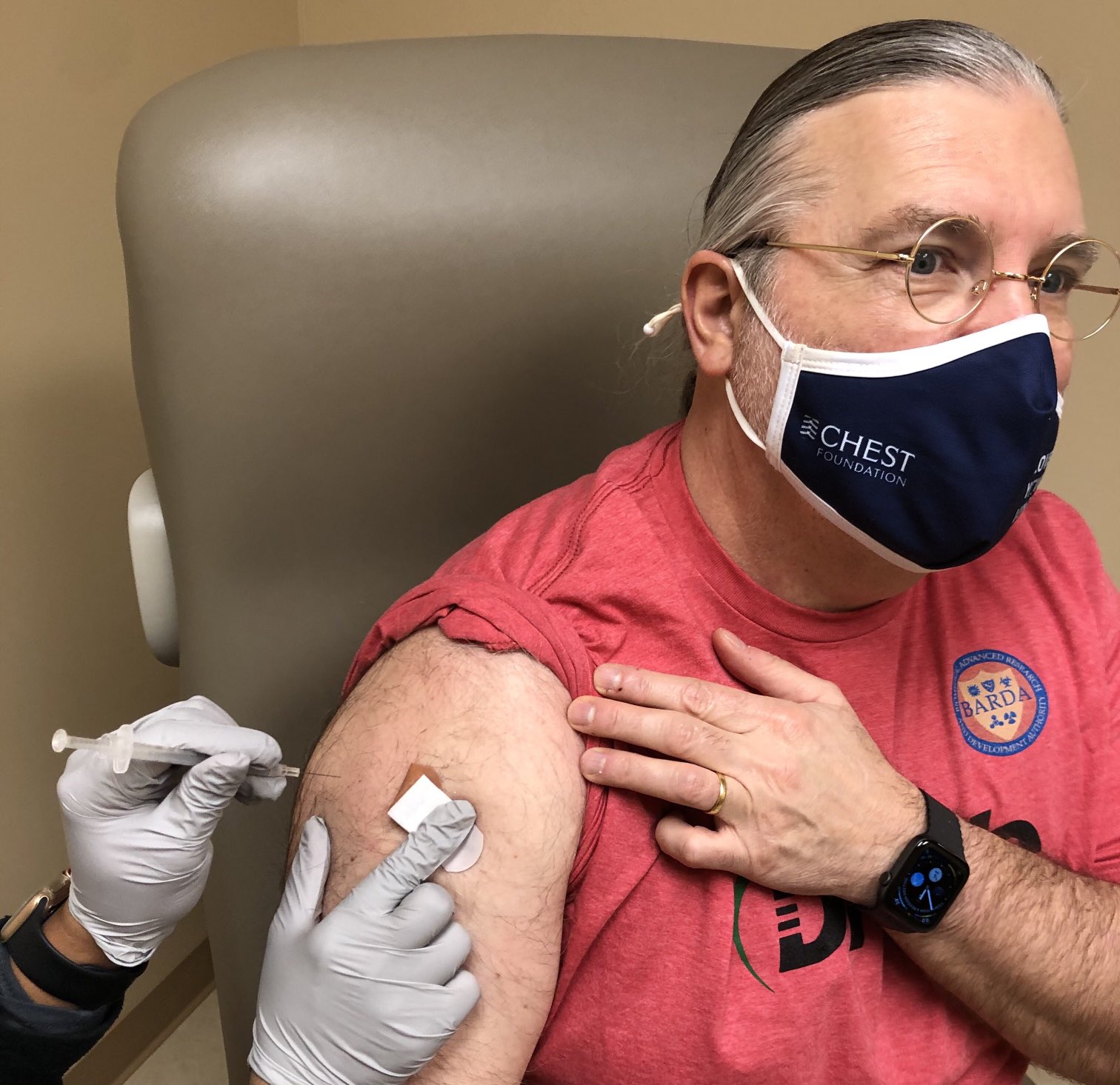
All roads lead to sepsis – including COVID-19. Our team members and frontline physicians, Dr. Timothy Buchman and Dr. Steve Simpson, received their first COVID-19 vaccine shot this week
Drs. Timothy Buchman and Steve Simpson, IPA and Senior Medical consultants to DRIVe’s Solving Sepsis program, receiving their first COVID-19 vaccine shot. ...(more)

Barracuda Bowl Pitch-Off
Watch unique startups pitch their medtech innovations to a panel of investors, and hear what they think about the technologies, development, and financial projection ...(more)

BARDA DRIVe seeks interested applicants for the Early Notification to Act, Control, and Treat (ENACT) program
On October 22, 2020, BARDA DRIVe reopened its EZ Broad Agency Announcement (EZ-BAA) solicitation for the ENACT program to support the development of novel physiological sensors ...(more)
November 2020

Empatica to deploy a COVID-19 early detection wearable
In June, we announced our partnership with U.S. Department of Health and Human Services (HHS) to validate he COVID-19 detection ...(more)
October 2020

Evidence of Long-range nerve pathways connecting and coordinating activity in secondary lymph organs
Peripheral nerve reflexes enable organ systems to maintain long-term physiological homeostasis while responding to rapidly changing ...(more)

BARDA seeks abstract submissions for medical countermeasure development through the DRIVe ReDirect Program
Chemical agents are poisonous vapors, aerosols, liquids, and solids that have toxic health effects. Chemical attacks could occur without warning and injury or death often occurs shortly after exposure ...(more)
September 2020

SBIR Phase I Award #2025773: A Wireless Portable Fully Electronic Hematology Analyzer for Complete Blood Count Analysis
The broader impact/commercial potential of this Small Business Innovation Research (SBIR) Phase I project is to develop a new device for...(more)
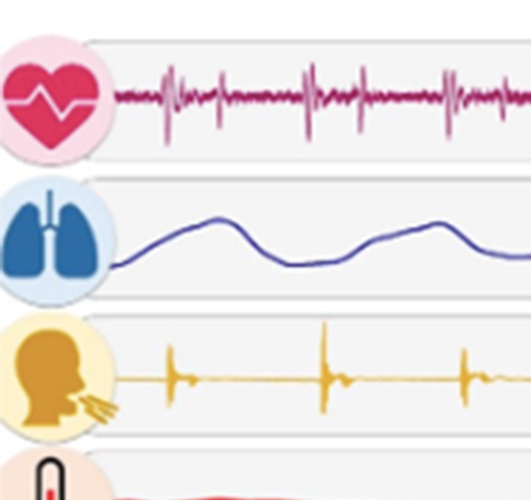
Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities
As of 20 June 2020, the Center for Disease Control’s tabulations show more than 2.2 million recorded cases of coronavirus. ...(more)
August 2020

98point6 Is Transforming Virtual Health Care with Text-Based Primary Care
Consumers today expect convenience in all aspects of their lives, including health care. Consumer Technology ...(more)

BARDA establishes four new partnerships to explore innovative vaccine delivery technologies
New vaccine delivery technologies could provide individuals with a broader range of options for immunization, including ones that are easier to use and potentially more effective than traditional vaccination ...(more)
June 2020

BARDA partners with Colorado State University’s Infectious Disease Research Center (IDRC) to further develop the SolaVAXTM platform to address COVID-19
BARDA is partnering with Colorado State University’s Infectious Disease Research Center (IDRC) to further develop SolaVAXTM. The SolaVAX TM platform uses a proprietary technology to inactivate a virus ...(more)

BARDA partners with MBio Diagnostics to develop an in vitro point-of-care serology test for the detection of human anti-SARS-CoV-2 antibodies from whole blood samples
BARDA and MBio Diagnostics are committed to a public-private partnership to develop a test for COVID-19. The MBio COVID-19 Serology Test ...(more)

BARDA and Empatica forge new partnership to develop an early monitoring platform to identify COVID-19 infection
BARDA and Empatica embark on a new partnership to develop and seek U.S. Food and Drug Administration’s Emergency Use Authorization for Aura, a wearable system and algorithm that can ...(more)

BARDA supports development of two automated antibody tests for COVID-19 by Ortho Clinical Diagnostics
BARDA is pleased to announce a partnership with Ortho Clinical Diagnostics to further develop two automated antibody tests for COVID-19. ...(more)

BARDA and Immunexpress Inc. expand partnership to evaluate a sepsis host-based laboratory diagnostic to triage COVID-19 patients
BARDA and Immunexpress Inc. are collaborating to determine the risk of sepsis in COVID-19 patients in the hospital emergency department or ICU. These studies will be used to support 510(k) Food and ...(more)

BARDA DRIVe and Sepsis Alliance to provide educational content on COVID-19 and Sepsis
Sepsis Alliance and BARDA’s Division of Research, Innovation and Ventures (DRIVe) are partnering to provide two high-quality, evidence-based education and training webinars ...(more)

BARDA, Snapdragon Chemistry Inc., demonstrate deployable domestic manufacturing technology for COVID-19 vaccine component
BARDA and Snapdragon Chemistry Inc. of Waltham, Massachusetts, will work together over the next year to develop and demonstrate an innovative continuous manufacturing platform ...(more)

Latest BARDA collaboration with OraSure Technologies Inc. aims to develop highly reliable, high-throughput diagnostic test for SARS-CoV-2 antibodies in oral fluids
BARDA and OraSure Technology, Inc. are partnering again, this time on development of an in vitro (laboratory) diagnostic to detect antibodies for the SARS-CoV-2 virus in oral fluids ...(more)

BARDA partners with Evidation Health to identify COVID-19 infections more quickly in healthcare workers and first responders using self-monitoring and wearable devices
BARDA is pleased to announce a partnership with Evidation Health of San Mateo, California, to monitor healthcare workers and first responders by leveraging de-identified, patient-generated health data...(more)

BARDA partners with Quidel Corporation to develop one-hour, mobile-integrated multiplex test on Sofia platform to detect SARS-COV-2 plus three additional respiratory viruses
BARDA and Quidel Corporation entered into a partnership to develop a rapid multi-analyte diagnostic test for use on the Sofia 2 assay system to detect the SARS-CoV-2 virus...(more)

BARDA announces partnership with Siemens Healthineers to develop automated total antibody test for COVID-19
BARDA entered into a partnership with Siemens Healthineers to accelerate development of the SARS-CoV-2 total antibody test. This laboratory-based total antibody test can be used to detect ...(more)
May 2020

BARDA and VitalConnect partner to monitor nursing home and COVID-19 patients for early indication of patient deterioration
BARDA is partnering with VitalConnect to assess the ability of a wearable device to remotely monitor individuals during the COVID-19 outbreak for changes in health deterioration...(more)
BARDA Names First DRIVe Director
BARDA is pleased to announce Sandeep Patel, Ph.D. as the first director of the Division of Research, Innovation and Ventures (DRIVe)...(more)
April 2020

Non‐invasive Targeted Gastric Vagal Complex Stimulation: Preliminary in Human Cutaneous Measures of Gastric Slow Wave Function
Gastric slow wave abnormalities have been associated with upper gastrointestinal motility disorders. Invasive studies in humans have ...(more)

BARDA and InBios International, Inc. partner to expedite development for serological tests to identify asymptomatic or recovered cases of COVID-19
BARDA and InBios International, Inc. are collaborating on the rapid development of the SCoV-2 Ab Detect™ Rapid Test as a point-of-care assay (test) to detect SARS-CoV-2 reactive antibodies in a blood samples...(more)

BARDA announces partnership expansion with Cytovale, Inc. for continued development of Cytovale’s rapid diagnostic system for sepsis as part of COVID-19 pandemic response
BARDA and Cytovale, Inc. are expanding their partnership for additional advanced research and development of a diagnostic system being designed to diagnose sepsis in less than 10 minutes...(more)

BARDA and Tangen Biosciences, Inc. entered into a public-private partnership to develop a rapid diagnostic test for SARS-CoV-2
BARDA and Tangen Biosciences, Inc. entered into a partnership to develop a rapid diagnostic test for use on the Tangen GeneSparkTM molecular diagnostic system to detect the SARS-CoV-2...(more)

BARDA supports Hememics Biotechnologies, Inc to develop a rapid antigen and antibody diagnostic to identify current or past SARS-CoV-2 infections in 60 seconds
BARDA is collaborating with Hememics Biotechnologies, Inc. on the development of a rapid, Bluetooth-connected SARS-CoV-2 diagnostic test. The test is being designed to detect SARS-CoV-2 antigen...(more)

BARDA and Vela Diagnostics USA, Inc. entered into a public-private partnership to develop a rapid diagnostic test for the COVID-19 pandemic
BARDA and Vela Diagnostics USA, Inc. are entering into a partnership to develop a rapid diagnostic test for use on two instrument platforms to aid in the detection of COVID-19 infections...(more)

HHS/BARDA supports DiaSorin, Inc to develop a fully automated serology test to detect novel coronavirus infections
BARDA is collaborating again with DiaSorin, this time to develop a clinical laboratory test to identify people who have been infected with the SARS-COV-2 virus but have recovered...(more)

HHS supports Nanomix, Inc to develop a rapid mobile diagnostic test to detect presence of current or past COVID-19 infection in patients
BARDA has partnered with Nanomix, Inc. on the development of a COVID-19 rapid mobile test to diagnose COVID-19 infections with results in as little as 15 minutes. The test detects the presence of SARS-CoV-2 antigen...(more)

BARDA is partnering with OraSure Technologies Inc. to develop first rapid at-home COVID-19 diagnostic test
BARDA and OraSure Technology, Inc. are working together on the development of a point-of-care (POC) test for SARS-CoV-2 that could bring testing closer...(more)

BARDA supports Luminex Corporation development of a second diagnostic test to detect novel coronavirus infections
BARDA has partnered again with Luminex Corporation, this time on the development of a
COVID-19 sample-to-answer diagnostic. The test detects the presence of COVID-19...(more)

BARDA collaborates with GenMark Dx on a rapid diagnostic test to detect COVID-19
BARDA is partnering with GenMark DX to develop an accurate, rapid coronavirus diagnostic
for use in clinical and hospital labs. BARDA’s support follows a March 19 decision by
the U.S. Food and Drug...(more)
March 2020

BARDA and Luminex Corporation partner to expedite development and EUA clearance for COVID-19 diagnostic test
BARDA and Luminex Corporation are collaborating on rapid development of the diagnostic
test called the NxTAG CoV Extended Panel for COVID-19...(more)

HHS, DoD collaborate with Mesa Biotech on rapid diagnostics to detect coronavirus infections
The Biomedical Advanced Research and Development Authority (BARDA), part of the HHS
Office of the Administration for Strategic Preparedness and Response (ASPR), will
provide
Mesa...(more)

BARDA partners with Qiagen to develop a rapid COVID-19 diagnostic test
The U.S. Department of Health and Human Services (HHS) will partner with Qiagen LLC of
Germantown, Maryland, to accelerate development of a diagnostic test for the 2019 novel
coronavirus...(more)

HHS collaborates with DiaSorin Molecular to develop a COVID-19 diagnostic test
The U.S. Department of Health and Human Services (HHS) is supporting the development of
a novel coronavirus diagnostic test by DiaSorin Molecular, LLC of Cypress, California...(more)

HHS Seeks Abstract Submissions for 2019-nCoV Diagnostics Development
As part of the U.S. government's ongoing public health response to the 2019 novel coronavirus (2019-nCoV), the U.S. Department of Health and Human Services (HHS) today announced the opening of an Easy Broad Agency Announcement (EZ-BAA) for development of 2019-nCoV......(more)
January 2020

BARDA DRIVe partners with Rizlab Health to enable home-based blood analysis for differentiation of bacterial vs. viral infections
BARDA DRIVe is pleased to announce a partnership with Rizlab Health of New Brunswick,
New Jersey, to develop a transformative technology to help diagnose and differentiate
viral or bacterial infections prior to symptom onset......(more)

BARDA DRIVe partners with GE and The Feinstein Institutes for Medical Research on ultrasound-based pre-symptomatic pathogen exposure detection
BARDA DRIVe is pleased to announce a partnership with GE Research of Niskayuna, New York, and The Feinstein Institutes for Medical Research of Manhasset, New York, to detect pre-symptomatic exposure to viral or bacterial pathogens using ultrasound. ......(more)
October 2019

HHS to advance development of Cytovale rapid sepsis diagnostic
To advance the development of a test that may be able to diagnose sepsis in less than 10 minutes, the U.S Department of Health and Human Services (HHS) will partner with Cytovale of San Francisco......(more)

HHS joins forces with Beckman Coulter on machine-learning algorithm to identify Sepsis
Identifying patients with sepsis in hospital emergency departments, intensive care units and other hospital settings could be done earlier with diagnostic tests based on machine-learning algorithms......(more)

DRIVe and the Rory Staunton Foundation partner to combat maternal sepsis
BARDA DRIVe is teaming up with the Rory Staunton Foundation for Sepsis Prevention to help combat maternal sepsis, one of the leading causes of pregnancy-related deaths......(more)
September 2019

DRIVe partners with 98point6 on technology for novel telemedicine approaches to treat infectious diseases and improve situational awareness for public health authorities
BARDA DRIVe is partnering with Seattle-based 98point6 to leverage its on-demand primary care smartphone application......(more)

DRIVe partners with Sound Life Sciences for home-based smart speaker respiratory monitoring of patients
BARDA DRIVe is pleased to announce a partnership with Sound Life Sciences (SLS) of Seattle, Washington. The startup company will deploy a contactless motion and respiratory monitoring......(more)
HHS expands network of innovation accelerators
Five accelerators selected to speed development of innovative medical products to transform U.S. health security......(more)
August 2019
BARDA DRIVe partners with Sonica Health on an advanced, bio-integrated sensor system to detect pre-symptomatic pathogen exposure
BARDA DRIVe is pleased to announce a partnership with Sonica Health of Evanston, IL to assess cardiopulmonary health. Sonica’s bio-integrated, wireless sensor technology comprises a thin, soft, electronic system that gently adheres to the skin......(more)
July 2019

DRIVe and Inflammatix partner to develop a host response-based diagnostic to identify and risk-stratify patients suspected of sepsis
BARDA DRIVe is teaming up with Inflammatix, Inc. to advance development of a host-response-based diagnostic (HostDxT™-Sepsis)...(more)

DRIVe partners with University of California San Diego
BARDA DRIVe is pleased to announce it is partnering with UC San Diego to develop a miniaturized Vagus Sentinel, Magnetometer (VS-M), a low-profile wearable...(more)
June 2019

DRIVe partners with Evidation on using data generated by wearable biomonitoring devices to predict respiratory infections and exposures to pathogens...
BARDA DRIVe is pleased to announce a partnership with Evidation Health of San Mateo, California, to leverage de-identified, patient-generated health data from everyday device......(more)
Join DRIVe's network of accelerators!
BARDA DRIVe seeks to expand our network of innovation accelerators into new areas of the country to support new product development to improve...(more)
May 2019

DRIVe partners with Janus-I Science to develop new diagnostic assay that can identify unknown viral pathogens in patient clinical specimens
BARDA’s DRIVe is partnering with Janus-I Science, Inc., of Vista, California, to develop a diagnostic assay that is capable of identifying unknown infectious disease pathogens in patient specimens. This transformative technology...(more)

BARDA DRIVe partners with Enesi Pharma to develop a solid dose vaccine delivery technology for easier administration of vaccines
BARDA’s DRIVe is partnering with Enesi Pharma, of Oxford, UK, to develop a transformative vaccine administrative technology. The ImplaVax® formulation and needle-free system enables solid dose implants containing vaccines...(more)
March 2019
DRIVe EZ BAA Portal Is Live
The BARDA DRIVe team is pleased to announce that the new, easy-to-use EZ BAA online Portal is now live. This Portal will replace our existing PDF-based process, helping to improve the user experience, data integrity, and...(more)
DRIVe Releases New Award Opportunity
The DRIVe Special Instructions are open now and will close on May 28, 2019. Medical product developers, research teams, and companies offering disruptive solutions to health security threats are invited to review...(more)
DRIVe partners with Qvella Corporation to develop an mRNA-based assay to rapidly identify infection and septic patients in under 1 hour
The BARDA DRIVe Solving Sepsis initiative is partnering with Qvella Corporation of Carlsbad, California, to develop ground-breaking technology to diagnose...(more)
February 2019

DRIVe partners with Empatica to develop its FDA-cleared Embrace2 smartband technology to predict illness prior to symptom onset
BARDA DRIVe is pleased to announce it is partnering with Empatica of Cambridge, Massachusetts, to apply Empatica's wearable biomonitoring device...(more)

DRIVe partners with Immunexpress to develop a medical diagnostic device to rapidly detect sepsis in patients
The DRIVe Solving Sepsis initiative has partnered with Immunexpress of Seattle, Washington, to develop ground-breaking technology that analyzes a patient’s immune system to diagnose...(more)
January 2019

New Sepsis Institute provides multiplatform sepsis education training programs for healthcare professionals
To educate healthcare providers on recognizing the signs of sepsis across the continuum of care, DRIVe and Sepsis Alliance, one of the nation’s leading sepsis organizations...(more)

DRIVe teams up with academic research consortium to develop deep learning software to provide early warning of sepsis in patients
The DRIVe Solving Sepsis initiative is partnering with Emory University to further validate an interoperable machine learning software for early prediction of sepsis in hospital intensive...(more)

DRIVe Partners with Biobeat
DRIVe partners with Biobeat Technologies of Israel to develop a wearable monitoring device that diagnoses the flu and infections before any symptoms appear...(more)
November 2018
DRIVe partners with Cytovale to develop a rapid diagnostic for sepsis
DRIVe is pleased to announce it is now partnering with Cytovale, Inc. of San Francisco to develop new technology...(more)
HHS is recruiting for a permanent Director to lead the new BARDA Division of Research, Innovation, and Ventures (DRIVe)
BARDA is seeking to recruit an exceptionally talented and forward-looking leader...(more)
September 2018
DRIVe awards InnaMed
DRIVe’s Solving Sepsis program is excited to partner with InnaMed of Philadelphia. InnaMed is developing...(more)
DRIVe announces its first Solving Sepsis award
The BARDA DRIVe Solving Sepsis program welcomes its first partner, Prenosis of Chicago, IL. Prenosis is developing ...(more)
DRIVe awards Spire
DRIVe welcomes partner Spire, Inc. of San Francisco. DRIVe is providing research and development funding for the new Spire Health Tag...(more)
DRIVe awards EnLiSense
DRIVe is excited to announce its first Powered by DRIVe Awardee: EnLiSense of Allen, TX. This partnership...(more)
2018

Timothy Buchman joins the DRIVe Solving Sepsis team
Timothy G. Buchman, MD has four decades of bedside experience caring for septic patients. He is Professor of Surgery, Anesthesiology...(more)

Steven Simpson joins the DRIVe Solving Sepsis team
Steven Q. Simpson, MD is a prominent medical researcher and expert in sepsis....(more)
DRIVe requests feedback for Incubators
DRIVe is seeking information from industry incubators operated or associated with a pharmaceutical, biotechnology and medical technology companies to help establish DRIVe’s new Fuel Laboratories...(more)

Tyler Merkeley appointed Interim Director of BARDA's DRIVe
This Division will adopt a bold new approach to partnering in order to accelerate life-saving innovation in medical products and...(more)

Apply now!
DRIVE's EZ-BAA application is streamlined,
fast and user-friendly
DRIVe's new application process, called an EZ-BAA, is now open for business! Medical product developers, research teams and...(more)